Stress response in plants comprises repertoire of molecular, cellular cross-talk and signaling responses initiated through the detection of specific or combined biotic or abiotic stress effect that may result in the induction of SM [58]. Plants immune system have evolved numerous stress detection mechanisms that includes transmembrane recognition (in response to evolving pathogen or microbial association molecular pattern), polymorphic NB-LRR protein production by most R-genes (to large extent inside cell) and production of SMs to cope with the stress situations, and thus, become remodeled to endure the condition [59, 60]. This may be achieved through influence on physiological processes in plant cells, triggered by signal transduction process to accommodate the stimulus involving adjustments in primary and SM that enable regulation of cell osmotic pressure, prevents cell components oxidation, pathogenic microbial growth and infection, and deter herbivores [11, 31, 60] through biochemical and physiological processes associated pathways regulation. Induction of the stress may stimulate expression or repression of stress-genes network through precise regulation that may result in the production of functional cellular molecules to accommodate the stress effect [61–63] which may be in the form of biosynthesis of osmoprotectants, detoxification enzymes, transporters, chaperones and proteases that serve as the first line of cellular protection [64]. In many instances, also, the stress response involves reversible salicylic and jasmonic acid production, ethylene and reactive oxygen species (ROS) production, ion fluxes, phosphorylation, promoter elements and transcription factors [65]. Recent evidences have shown the role of regulatory proteins activation and signaling molecules in regulating signal transduction processes and expression of stress-responsive genes as an early response that prevents cellular damage and re-establish homeostatic state essential for growth of plants during in vitro and in vivo stress growth conditions [11, 64, 66–68]. However, knowledge about the effect of stress on the PSM is to a large extent, based on research efforts towards yield maximization of bioactive constituents from herbs, spices, and medicinal plants through evaluating tissue or organ physiology while understanding cellular functional role the metabolites are playing in plant cells is stimulating interests, particularly on the production during in vivo and in vitro growth. On the other hand, defense response system involves production of an array of chemical, structural and protein-based physio-molecular response(s) against invading foreign body or organism into plantsˈ systems through variety of responses found in various plant taxonomic groups [20, 22, 26, 69]. The defense response system becomes activated when intra- or extracellular signal is received by receptors in cell plasma membrane, involving their binding accompanied by signal transduction cascade initiation that may result in de novo synthesis or activation of transcription factors responsible for regulating SMs biosynthesis genes expression [23]. This may lead to systemic adjustment and, in many instances, a diseased condition or even becomes regular part of physiological processes [21, 31]. Of importance in the defense response system is the perception of the stress that leads to initiation of efficient recognition and basal defensive mechanism for activation of different signaling cascades associated with a given stress effect [31, 70, 71]; defensive response may be constitutive or induced with the former and its secondary compounds always present in plant, and often species-specific in existence in the form of stored compounds, precursors of active compounds that may be easily activated in response to damage caused on plant body or conjugated compounds [21, 72]. The latter gets initiated after the actual damage occurs on plant body and may involve production of defensive proteins that includes lectins and protease inhibitor(s) or production of toxic SMs [21]. Recent understandings suggest that induction of a defense response system may involve wounding and recognition of elicitor compounds that could lead to trigger of signaling pathways and resultant initiation of action at distant region of plant [26]. For instance phytoalexins are produced by many plant species in response to microbial invasion to serve defensive response function. Similarly, production of isoflavonoid phytoalexins in soybean and alfalfa and sesquiterpenes by members of the family Solanaceae is another example of their defensive function. Overall, both stress and defense response processes stimulate metabolic changes that may result in the biosynthesis of bioactive compounds having pharmaceutical or nutritional value.
Plant secondary metabolites could be detected in cells of the whole plant body but, site of biosynthesis, in most of the cases, is restricted to an organ and transported to different region through vascular tissues or symplastic and apoplastic transport to the site of storage, depending on polarity of the metabolite [35, 73]. Hydrophilic compounds that include alkaloids, glucosinolates and tannins are stored in vacuoles or idioblasts whereas lipophilic such as terpene-based essential oils could be stored in thylakoid membranes or cuticles, resin ducts and trichomes [73, 74]. The sites or storage tissues and structures may include leaves, shoots, roots, flowers, callus or somatic embryos and specialized accumulation sites such as glandular trichomes, periderms, and phellem among others. For instance, monoterpenes produced by members of Labiatae are biosynthesized in secretory cells but, become accumulated in epicuticular cavity of glandular trichomes [75]. In the past recent decades, it has been established that spatial and temporal change in function related to production of the PSMs in many storage sites could be encountered, based on the growth physiology and developmental stage of plant species investigated [35, 51–53, 76–79]. Thus, accumulation of a SM in plant at higher levels could be an indicator of high expression of genes and metabolic pathway for its biosynthesis in cells, although translocation of a bioactive compound from site of biosynthesis to storage site plays significant role with some of the PSMs [1, 80]. For instance, involvement of membrane transportation system through ATP-binding cassette (ABC) transport has been implicated in the accumulation of PSMs in many medicinal plants [81]. In a study aimed at understanding the kinetics of berberine production and storage in the cell cultures of Coptis japonica, Sato et al. [82] demonstrated concentration gradient-based uptake of the alkaloid when added into the culture medium of cultured cells of the species, and its subsequent accumulation in vacuole of cells. Transport of the alkaloid involved uptake at the levels of plasma membrane and subsequent efflux of berberine in cytosol and into vacoular lumen at tonoplast levels. Recent evidences on production of some PSMs to specific structures in plant body have also implicated their protective functional role through defense response in the growth environment [26, 73]. Of worthy note is the fact that much of the above information and understandings about the role of stress and defense responses in PSMs production either involves application of plant cell culture or pot experiments [83–85], with medicinal plant Catharanthus roseus as the model species widely investigated for production of its anticancer alkaloid vincristine and vinblastine [86]. Over three decades ago, Wink [87] suggested that production and concentration of a SMs produced by a plant species is determined by equilibrium relationship between biosynthesis, storage, and degradation, based on the stage of development as to which becomes dominant. In many recent literature reports, it had indeed been shown that an array of responses involving signal transduction systems and molecules with influence on tight regulation of biosynthetic pathway(s) could be characterized in different plant species, genotype or cultivars due to ecotype and genetic component-dependent variations response to the stress or defense response function during growth condition(s) of plants. This influences SM, depending on the season, environmental or external triggers [8, 22, 26, 73]. For example, production of essential oils in the trichome of leaves imply their defensive role against herbivores or insect predators while tannins production in the vacuoles of leaves cells located beneath epidermal surface and their bitter taste deter predators [73, 88]. Over 3 decades ago, Wink [87] also opined that higher accumulation of alkaloids in the seeds of most plants could be considered a chemical defensive strategy, and for use as source of nitrogen during germination. Competition with microbes and other essential mineral nutrients, defensive pathogens and herbivores attack to plants may induce SM pathways in plant cells [21, 50]. This may involve hypersensitive response that leads to localizing an invading pathogen by plant system at the infection site, with phenolic-storing cells playing vital role in programmed cell death [89, 90]. Serotonin reported from many biota is believed to be involved in various physiological processes in many plants through protection from environmental stresses and against pathogenic invasion, as well as a role in scavenging ROS that leads to delayed senescence [6, 91]. It was reported to serve protective function from environmental stress in the reproductive tissues of young Datura metel through antioxidant role, and exposure of flowers to cold stress significantly enhanced the production [92]. Polyamines, spermidine, spermine and putrescine found in wide range of biota are involved in variety of physiological processes that include senescence, development and stress responses [93]. Production of the polyamines at higher cellular levels by plants is associated with tolerance to environmental stresses. Thus, stress-tolerant plants possess high capacity for their enhanced biosynthesis during abiotic stress growth conditions, and certain polyamines could act as elicitors to the production of PSMs [93]. Xanthophyll that contains conjugated double bonds in their long chain is involved in xanthophyll cycle, and performs the function of excess light dissipation into harmless heat energy in plant cells [94] while phenolics storing cells play a vital role in the development of programmed cell death [90]. Although the exogenous application of jasmonates to the plants had been proven to cause morphological and physiological effects, they are also associated with the production of PSMs that form an integral part of the defense responses [95]. Their application stimulated biosynthesis of many SMs in the cell cultures and intact plant species [95]. Flavonoids, phenolics and polyphenolics are ascribed significant role in plant antioxidant responses and development, pigment and lignin biosynthesis [96]. The above examples have shown few among the enormous SMs and signaling molecules produced by plants in response to the stress or defense signal function, and variation within genotype, taxonomic group and physiology, and experimental technologies employed in their evaluation is variable. Similarly, the metabolites perform varied physiological cellular functions essential for growth of the plants at varied degree. However, it is still difficult to ascertain their stress and defensive function given the poor understanding of cellular level functions and spatial and temporal changes encountered in the production, based on experimental approach employed. Additionally, demarcation on the production of a specific metabolites in response to the stress and defense response function is difficult, and as such, ascertaining the existence of interconnection between primary and secondary metabolic pathways that provide precursors to the SM pathways in plant cells is difficult. In many recent studies, it had been shown that SM system in plants is a response to the stress and defensive situations that leads to an enhanced biosynthesis of the metabolites in an integrated defense mechanism through dynamic ways (e.g. Tables 1 and 2). However understanding the signaling processes involved and their interconnection with the primary metabolism is yet unclear, and very few had been investigated in some taxonomic groups, based on plant tissues or organs evaluated with rare reports on whole plant system evaluation or cellular levels.
Table 2.Production of some plant secondary metabolites under various in vivo growth condition of plants
Secondary metabolitePlant source(s)Tissue analyzedGrowth conditionReference(s)ArtemisininArtemesia annuaWhole seedling (treated and control)Salt, drought and water logging[350]CamptothecinCamptotheca acuminataSeedlingsNitrogen, drought and anti-transpiration agents[125, 128]CodeinePapaver somniferumPlantletsDrought stress[260]Rosmarinic acidSalvia miltiorrhizaLeaves, roots and aclimatized plantlet shootsHydroponic culture[351]RohitukineDysoxylum binectariferumSeedling (roots, collar region of stem and young leaves)Normal[352]SteviosideStevia rebaudianaLeaves (dried)Hydroponic culture, salt stress[353–355]AllicinAllium sativumWhole plantPot experiment on light effect[356]AndrographolideAndrographis paniculataLeaves and stemOpen field experiment with plant populations[357]ResveratrolGrapes, GroundnutLeaves, shoot, roots and whole plantNumerous[358, 359]Betalain pigmentsCaryophyllales membersDifferent plant partsDifferent growth condition[360]SaikosaponinsBupleurum chinense1-year-old plants, plantsDrought, watering and re-watering, fertilization[294, 361]Hyoscyamine and scopolamineAtropa belladonna extracts32-week-old dried rootIrrigation in greenhouse experiment[362]CapsaicinCapsicum sp.FruitsSalinity-induced stress[363]SennosidesCassia augustifoliaPre-, post and flowering plantsPot culture experiment[364]Indole alkaloidsCatharanthus roseousLeavesGreenhouse under binary stress-induced condition[365]Asiaticoside and madecassosideCentella asiaticaLeaves (post-harvest)Low temperature and water dehydration[366]ValepotriatesValeria speciesAll organsNormal growth condition (Iran)[367]RutinDimorphandra mollisAll plant parts at different growth stagesNormal, drought, flooding and salinity[368]FuranocoumarinsBituminaria bituminosaLeaves dry matter and fruitsField conditions and hydroponics[369]GlycyrrhyzinGlycyrrhyza glabraPlants at seedling and adult stage, stolonsDrought stress[370]Zealexins and kauralexinsMaizeRootsDrought stress[371]Open in a new tabLiterature reports in the past decades, had shown that during in vivo growth condition of plants, adverse environmental stress and climatic factors that includes drought, temperature extremes (freezing and heat), light irradiance, nutrients deficiency and soil contamination with high concentrations of ions (metals and salts) are main stressors that influence plant physiology (Fig. 1) with stimulatory effect on SM in crops and medicinal plants [6, 97–103]. Similarly, the conditions of in vitro culture imposes a combination of stress factors to cultured plant cells through pronounced change in cellular environment that may be in the form of wounding of excised tissues, plant growth regulators (PGRs), salt concentrations (low or high) and high or low artificial light levels that could generate stress effects. This may lead to the induction of SM pathways, depending on the physiological state of plant cells [10, 55, 104]. Hence, of all the in vitro techniques applied in PSMs production, elicitation—which is based on the principle of stress induction, is the most effective strategy for enhancing production of the metabolites through the use of biotic and abiotic elicitors that promote biosynthesis of the molecules when added into culture medium during cultivation of plant cells, tissues and organs [13, 38, 55, 57, 105]. Further, because in coping with the in vivo and in vitro stress challenges, plants have evolved efficient mechanisms for recognition and adaptation to the elicitation, it indeed influences plant physiology and biosynthesis of the metabolites. This may involve adjustments in photosynthetic rates, stomatal conductance and transpiration (in vivo), cell wall architecture, membrane systems, alterations in cell cycle and division rates (Fig. 1) with overall effect on general growth to fine-tune physiology and metabolism of bioactive compounds [106, 107]. It may involve expression or repression of gene regulatory network in response to the stress effect(s) [61–63] to confer tolerance at cellular levels by producing tolerance-associated molecules essential for regulation of signal transduction systems and stress responses [64, 68]. For example, production of flavonoids and cinnamic acid derivatives during drought-induced stress tolerance in cotton suggests their high efficiency in ROS scavenging [108] while isoprenes production due to heat-induced stress indicates their effective oxygen quenching antioxidant capacity in reed plants [109–111] Phenylamides are produced for efficient quenching of singlet oxygen radicals in plant cells during stress [112] while phenylamines accumulation in tobacco and bean due to abiotic stress suggests their antioxidant role [10, 113, 114]. Flavonoids, terpenoids, and volatile secondary metabolites provide color and scent properties to plants, which entails repellent and attraction effects on insects and herbivores, while toxins could be involved in plant-plant allelopathic effects [10, 115]. Generally, during both stress and defense response in plant cells, the fixed carbon through photosynthesis becomes allocated to SM with an overall effect on growth inhibition (Fig. 1), and synergistic effect may be encountered in some plant systems [20, 116]. For instance, biosynthesis of phenylpropanoid SMs in tobacco showed regulated control by carbon–nitrogen status and the production was confirmed by gene expression studies [117]. Combined effects of pest with the abiotic stress promoted production of SMs in cotton [118] while combination of wounding with water-induced stresses showed synergistic action in the production of phenylpropanoid SMs in carrot [119]. However, this depends on the sampled plant species, cultivation season, genotype and cultivar investigated [7, 8, 101, 120, 121]. The differences can be ascribed to the cellular receptor specificity, subcellular localization of ROS production, specific and regulation of MAPK activities, existence of differences in the activation of genes and product of their expression among plant species, genotypes and cultivars, and in relations to the inducing environmental factors [24, 122, 123].
Fig. 1.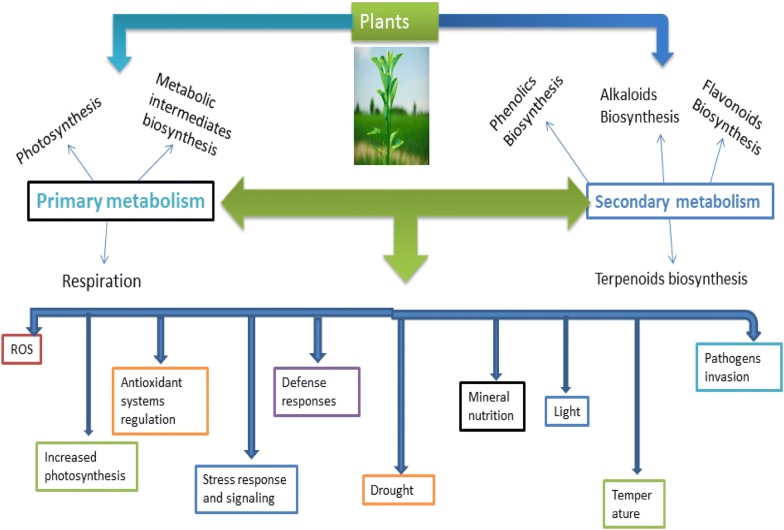
In their natural and in vitro growth conditions, plants encounter variety of stresses and biotic disturbances which leads to the initiation of stress and defense responses mediated by signaling processes and pathways involving repertoire of molecules to perform cellular functions essential for physiological processes. The physiological processes impact primary metabolism that provides biosynthetic intermediates for secondary metabolism, with concomitant effect on biomass and bioactive compounds biosynthesis. This generally depends on the species, genotype and cultivar as well as the stage of development and physiological state of the plant investigated